Dr Miguel Ramirez Moreno studies tau through his work as a postdoctoral researcher with Professor Amrit Mudher, through her AS funded project grant. Miguel has put together this helpful explainer on tau's function in dementia.
What is tau?
Tau is a protein naturally produced by our cells, helping them function properly. Its roles include supporting the internal structure of cells - by acting as a kind of strengthening scaffolding - to make them stronger.
This is especially important for our neuron cells, as they need to be made up of strong and resilient parts, to communicate with each other, transporting everything from electric signals to vital nutrients
Good maintenance by proteins like tau keeps our bodies working efficiently. However, tau has gained a negative reputation because it is involved in several diseases that lead to dementia, including the most common one, Alzheimer’s disease.
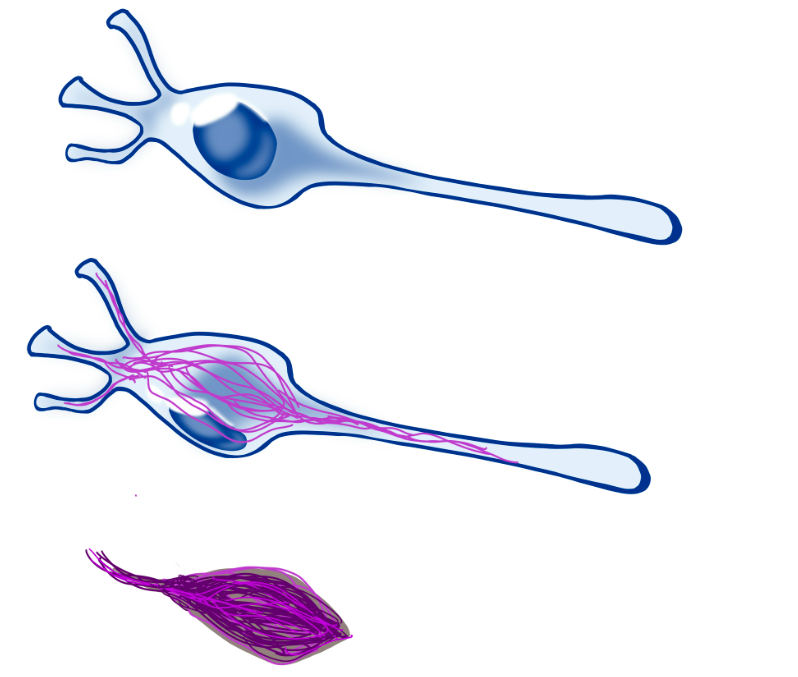
In the image above, we see a healthy neuron (top). tau acts normally across the structure of a neuron, but abnormal tau starts forming fibrous tangles (centre), that eventually are all what remains of the neuron (bottom).
Did you know... We all have tau inside our brains – inside every one of our cells. And normally that is fine. The problem is not the protein itself, but its behaviour.
Untangling findings on tau and Alzheimer’s disease
Dementia is not a single disease, but a syndrome caused by many different conditions affecting the brain. Alzheimer’s disease is responsible for more than half of all the diagnosed cases of dementia.
The disease was first spotted over a century ago, by Alois Alzheimer. He described the strange clumps he’d seen inside of the brains of people affected - fibre-like structures, or “tangles”.
As is often the case with major scientific breakthroughs, it took a collaborative effort to understand what these fibres actually were.
In 1975, a group at Princeton University (USA) discovered tau as a protein involved in protecting the internal structure of the cells. But it was not until 1988 that several scientists at Cambridge University (UK) found out that tau was making the tangles that Alzheimer described decades earlier.
The mystery of the tangles Alois Alzheimer had seen was solved: Finding tau was present in a form it should not be in Alzheimer’s disease. But this opened up more questions for researchers of Alzheimer’s disease. what Was this just an unusual presentation or something more? Could tau be responsible of for damaging our neurons getting hurt?
How relevant is tau for dementia?
Alzheimer’s disease is marked by the presence of abnormal tau tangles, alongside excessive depositions of amyloid beta – commonly known as senile plaques.
Scientists have discovered many ways in which these two proteins interact in the disease process. According to the amyloid cascade hypothesis, amyloid beta is disrupted first, which subsequently leads to changes in tau and other proteins. Abnormal tau is also linked to many other forms of dementia, either on its own or in combination with other proteins. This is why these conditions are collectively referred to as ‘tauopathies’. In each of these diseases, tau may be more or less important, but it is always involved.
What do and don’t we know about tau?
When tau protein becomes abnormal, it stops doing its usual job of keeping brain cells healthy. This leaves the cells more vulnerable to damage. But it doesn’t just stop helping, it can start harming the brain.
Scientists have found that abnormal tau can move from one neuron to another, which helps explain how the damage spreads across the brain in diseases like Alzheimer’s.
What research are Alzheimer's Society supporting into tau?
Research will beat dementia and will lead to improved diagnosis, effective treatments and the high-quality care that everyone living with dementia deserves. Learn more about some of the research we are funding to answer the outstanding questions about the tau protein, and how it could be targeted by future treatments.


Susan beechall
says
Alzheimer's Society
saysHi Susan,
We’re not running any trials ourselves but if you're interested in taking part in clinical trials in general then you can through the join dementia research service: www.joindementiaresearch.nihr.ac.uk.
Best wishes,
The Alzheimer's Society research team
lyn cullum
saysHarry Spinks
says
Alzheimer's Society
saysHi Harry,
Thank you for your comment. The appointments with your neurologist sound really difficult.
You might want to get in touch with our Dementia Support Line on 0333 150 3456. We can offer advice and help for you and your wife.
You can find out more about the Support Line and how our Advisers can help on this webpage: https://www.alzheimers.org.uk/get-support/dementia-support-line.
Best wishes,
The Alzheimer's Society web team
Zee
says
Alzheimer's Society
saysHi Zee,
At the moment, there isn’t an approved treatment which would target abnormal tau. But researchers and pharmaceutical companies are actively working on developing tau-specific therapies for the future. You can find more information on this webpage: https://www.alzheimers.org.uk/what-we-do/researchers/news/researching-new-drugs-alzheimers-disease.
Best wishes,
The Alzheimer's Society research team
Susan
saysMohammed Y Ali
saysEmma Pearson
saysJonh
says
Alzheimer's Society
saysHi Jonh,
I’m so sorry to hear about your mother. I’m afraid it’s very hard to be able to predict with any confidence how quickly someone will progress – both in terms of how quickly their symptoms get worse and how long they will survive with their condition.
We do have information on progression in dementia, if you’re interested in knowing more: https://www.alzheimers.org.uk/about-dementia/stages-and-symptoms/progression-stages-dementia
However, other than average survival times after diagnosis, which are very much just averages with some people declining much quicker and others much slower, we can’t give you an answer that would allow you to plan for any kind of individual timeline. It’s one of the most difficult parts of supporting someone with dementia.
Best wishes,
The Alzheimer's Society knowledge team
Mary beal
says
Alzheimer's Society
saysHi Mary,
Thank you for your comment. This sounds so incredibly difficult.
You might want to get in touch with our Dementia Support Line on 0333 150 3456. We can offer advice and help for you and your husband.
You can find out more about the Support Line and how our Advisers can help on this webpage: https://www.alzheimers.org.uk/get-support/dementia-support-line.
Best wishes,
The Alzheimer's Society web team
Howard Deacon
saysKayleigh
saysClare Virginia Heather
says
Alzheimer's Society
saysHi Clare Virginia Heather,
Thanks for your comment. You can find out more about Alzheimer's disease on this webpage: https://www.alzheimers.org.uk/about-dementia/types-dementia/alzheimers-disease
You might also want to get in touch with our Dementia Support Line on 0333 150 3456. You can find out more about the Support Line and how our Advisers can help on this webpage: https://www.alzheimers.org.uk/get-support/dementia-support-line.
Best wishes,
The Alzheimer's Society web team
Marilyn Williams
saysRaquel
saysLiza Sentance
says
Alzheimer's Society
saysHi Liza,
Yes, there are links between epilepsy and dementia.
People who have a history of seizures, particularly if they’ve struggled to control them, are more likely to have problems with their memory as they get older.
Sometimes this can develop into Alzheimer’s or other types of dementia. It also works the other way around. People with Alzheimer’s disease are much more likely to develop seizures as their condition progresses.
We have a blog post on the topic, if you would like to know more: https://www.alzheimers.org.uk/blog/what-link-between-seizures-and-dementia
Best wishes,
The Alzheimer's Society knowledge team
Sherry Jackson
says
Alzheimer's Society
saysHi Sherry,
It’s great to hear that you’re managing to adjust well to your frontotemporal dementia. It’s definitely a good thing to keep as active and engaged as you can.
Frontotemporal dementia would only be detectable at birth if it was caused by a disease gene, which sometimes it can be.
But knowing if you or your child carries an FTD disease gene is a very difficult decision for most people to make. Ideally, it’s one they should make when they’re adults and can decide if they want to live with that knowledge.
Best wishes,
The Alzheimer's Society knowledge team
Jacintha Manchester
says
Alzheimer's Society
saysHi Jacintha,
Both! We're funding research to understand the causes of dementia, develop effective treatments, improve care and ultimately find a cure. Understanding how and why tau behaves abnormally is important to develop potential therapies that would stop these events, which is a current focus of many research projects we fund.
Best wishes,
The Alzheimer's Society research team